Radium chloride
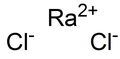 |
|
| Identifiers | |
|---|---|
|
10025-66-8 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
20138060 |
| ECHA InfoCard | 100.030.020 |
| UNII |
KKO873WR2Z |
|
|
|
|
| Properties | |
| RaCl2 | |
| Molar mass | 296.094 g/mol |
| Appearance | Colorless solid |
| Density | 4.9 g/cm3 |
| Melting point | 900 °C (1,650 °F; 1,170 K) |
| 245 g/L (20 °C) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Radium chloride (RaCl2) is a chemical compound of radium and chlorine, and the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.
Radium chloride crystallises from solution as the dihydrate. It may be dehydrated by heating to 100 °C in air for one hour followed by 5 1⁄2 hours at 520 °C under argon. If the presence of other anions is suspected, the dehydration may be effectuated by fusion under hydrogen chloride.
Radium chloride can also be prepared by heating radium bromide in a flow of dry hydrogen chloride gas, or by dehydrating radium sulfate with dry air and then heating the sulfate in a stream of hydrogen chloride.
Radium chloride is a colorless-white salt with a blue-green luminescence, especially when heated. Its color gradually changes to yellow with aging, whereas contamination by barium may impart a rose tint. It is less soluble in water than other alkaline earth metal chlorides – at 25 °C its solubility is 245 g/L whereas that of barium chloride is 307 g/L, and the difference is even larger in hydrochloric acid solutions. This property is used in the first stages of the separation of radium from barium by fractional crystallization. Radium chloride is only sparingly soluble in azeotropic hydrochloric acid and virtually insoluble in concentrated hydrochloric acid.
...
Wikipedia
