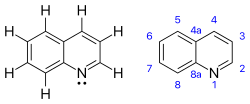
Quinolines
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Quinoline
|
|||
Systematic IUPAC name
|
|||
Other names
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 3DMet | B00959 | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.865 | ||
| EC Number | 202-051-6 | ||
| KEGG | |||
| MeSH | Quinolines | ||
|
PubChem CID
|
|||
| RTECS number | VA9275000 | ||
| UNII | |||
| UN number | 2656 | ||
|
|||
|
|||
| Properties | |||
| C9H7N | |||
| Molar mass | 129.16 g/mol | ||
| Appearance | yellowish oily liquid | ||
| Density | 1.093 g/mL | ||
| Melting point | −15 °C (5 °F; 258 K) | ||
| Boiling point | 237 °C (459 °F; 510 K) /760 mm Hg, 108 to 110 °C/11 mm Hg | ||
| Slightly soluble | |||
| Solubility | Soluble in alcohol, ether, and carbon disulfide | ||
| Acidity (pKa) | 4.85 (conjugated acid) | ||
| -86.0·10−6 cm3/mol | |||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
174.9 kJ mol−1 | ||
| Hazards | |||
| R-phrases | R21, R22 | ||
| S-phrases | S26, S27, S28, S29, S30, Template:S31, Template:S32, S33, Template:S34, S35, S36 | ||
| NFPA 704 | |||
| Flash point | 101 °C (214 °F; 374 K) | ||
| 400 °C (752 °F; 673 K) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
331 mg/kg | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
Quinoline was first extracted from coal tar in 1834 by Friedlieb Ferdinand Runge; he called quinoline leukol ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, strychnine, or cinchonine with potassium hydroxide; he called the compound Chinoilin or Chinolein. Runge's and Gephardt's compounds seemed to be distinct isomers because they reacted differently. However, the German chemist August Hoffmann eventually recognized that the differences in behaviors were due to the presence of contaminants and that the two compounds were actually identical.
Like other nitrogen heterocyclic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites. Owing to its relatively high solubility in water, quinoline has significant potential for mobility in the environment, which may promote water contamination. Quinoline is readily degradable by certain microorganisms, such as Rhodococcus species Strain Q1, which was isolated from soil and paper mill sludge.
...
Wikipedia