Prednisolone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | many |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682794 |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Biological half-life | 2-3 hours |
| Excretion | urine |
| Identifiers | |
|
|
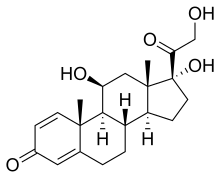
| Synonyms | 11,17-Dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydrocyclopenta[a] phenanthren-3-one |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.020 |
| Chemical and physical data | |
| Formula | C21H28O5 |
| Molar mass | 360.444 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Prednisolone is a steroid medication used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers. Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, and multiple sclerosis. It is used by mouth, injection into a vein, as a skin cream, and as eye drops.
Side effects with short term use include nausea and feeling tired. More severe side effects include pyschiatric problems, which may occur in about 5% of people. Common side effects with long term use include bone loss, weakness, yeast infections, and easy bruising. While short term use in the later part of pregnancy is safe, use long term or early in early pregnancy is occasionally associated with harm to the baby. It is a glucocorticoid made from hydrocortisone (cortisol).
Prednisolone was discovered and approved for medical use in 1955. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. It is available as a generic medication. The wholesale cost in the developing world is about 0.01 to 0.02 USD per day.
...
Wikipedia
