Potassium permanganate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Potassium manganate(VII)
|
|
| Other names
Potassium permanganate
Chameleon mineral Condy's crystals Permanganate of potash Hypermangan |
|
| Identifiers | |
|
7722-64-7 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
22810 |
| ECHA InfoCard | 100.028.874 |
| EC Number | 231-760-3 |
| KEGG |
D02053 |
| PubChem | 516875 |
| RTECS number | SD6475000 |
| UN number | 1490 |
|
|
|
|
| Properties | |
| KMnO4 | |
| Molar mass | 158.034 g/mol |
| Appearance | purplish-bronze-gray needles magenta–rose in solution |
| Odor | odorless |
| Density | 2.703 g/cm3 |
| Melting point | 240 °C (464 °F; 513 K) (decomposes) |
| 6.4 g/100mL (20 °C) 250 g/L (65 °C) |
|
| Solubility | decomposes in alcohol and organic solvents |
| +20.0·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.59 |
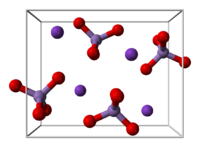
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
| 119.2 J/mol K | |
|
Std molar
entropy (S |
171.7 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−813.4 kJ/mol |
|
Gibbs free energy (ΔfG˚)
|
-713.8 kJ/mol |
| Pharmacology | |
| D08AX06 (WHO) V03AB18 (WHO) | |
| Hazards | |
|
EU classification (DSD)
|
|
| R-phrases | R8, R22, R50/53 |
| S-phrases | (S2), S60, S61 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
1090 mg/kg (oral, rat) |
| Related compounds | |
|
Other anions
|
Potassium manganite Potassium manganate |
|
Other cations
|
Sodium permanganate Ammonium permanganate |
|
Related compounds
|
Manganese heptoxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Potassium permanganate is an inorganic chemical compound and medication. As a medication it is used for cleaning wounds and dermatitis.
It has the chemical formula KMnO4 and is a salt consisting of K+ and MnO−
4 ions. It is a strong oxidizing agent. It dissolves in water to give intensely pink or purple solutions, the evaporation of which leaves prismatic purplish-black glistening crystals. In 2000, worldwide production was estimated at 30,000 tonnes. In this compound, manganese is in the +7 oxidation state.
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system. The wholesale cost in the developing world is about 0.01 USD per gram of powder. In the United Kingdom a 400 milligram tablet costs the NHS roughly £0.51.
Almost all applications of potassium permanganate exploit its oxidizing properties. As a strong oxidant that does not generate toxic byproducts, KMnO4 has many niche uses.
As an oxidant, potassium permanganate can act as an antiseptic. For example, dilute solutions are used to treat canker sores (ulcers), disinfectant for the hands and treatment for mild pompholyx, dermatitis, and fungal infections of the hands or feet.
Potassium permanganate is used extensively in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide (rotten egg smell) from well water via a "Manganese Greensand" Filter. "Pot-Perm" is also obtainable at pool supply stores and is used additionally to treat waste water. Historically it was used to disinfect drinking water and can turn the water pink. It currently finds application in the control of nuisance organisms such as zebra mussels in fresh water collection and treatment systems.
...
Wikipedia

