Polytetrafluroethylene
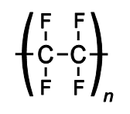 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
poly(1,1,2,2-tetrafluoroethylene)
|
|
| Other names
Syncolon, Fluon, Poly(tetrafluoroethene), Poly(difluoromethylene), Poly(tetrafluoroethylene)
|
|
| Identifiers | |
| Abbreviations | PTFE |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.120.367 |
| KEGG |
|
| Properties | |
| (C2F4)n | |
| Density | 2200 kg/m3 |
| Melting point | 600 K 327 °C |
| Thermal conductivity | 0.25 W/(m·K) |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
|
|
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. The best known brand name of PTFE-based formulas is Teflon by Chemours. Chemours is a spin-off of DuPont Co., which discovered the compound in 1938.
PTFE is a fluorocarbon solid, as it is a high-molecular-weight compound consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons demonstrate mitigated London dispersion forces due to the high electronegativity of fluorine. PTFE has one of the lowest coefficients of friction of any solid.
PTFE is used as a non-stick coating for pans and other cookware. It is very non-reactive, partly because of the strength of carbon–fluorine bonds, and so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubricant, PTFE reduces friction, wear and energy consumption of machinery. It is commonly used as a graft material in surgical interventions. Also, it is frequently employed as coating on catheters; this interferes with the ability of bacteria and other infectious agents to adhere to catheters and cause hospital-acquired infections.
PTFE was accidentally discovered in 1938 by Roy Plunkett while he was working in New Jersey for DuPont. As Plunkett attempted to make a new chlorofluorocarbon refrigerant, the tetrafluoroethylene gas in its pressure bottle stopped flowing before the bottle's weight had dropped to the point signaling "empty." Since Plunkett was measuring the amount of gas used by weighing the bottle, he became curious as to the source of the weight, and finally resorted to sawing the bottle apart. He found the bottle's interior coated with a waxy white material that was oddly slippery. Analysis showed that it was polymerized perfluoroethylene, with the iron from the inside of the container having acted as a catalyst at high pressure. Kinetic Chemicals patented the new fluorinated plastic (analogous to the already known polyethylene) in 1941, and registered the Teflon trademark in 1945.
...
Wikipedia

