Pixantrone
 |
|
| Names | |
|---|---|
|
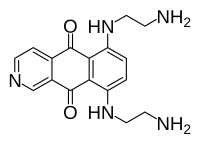
IUPAC name
6,9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5,10-dione
|
|
| Identifiers | |
|
784209-05-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL167731 |
| ChemSpider |
118174 |
| 7544 | |
| KEGG |
D05522 |
| PubChem | 134019 |
| UNII |
F5SXN2KNMR |
|
|
|
|
| Properties | |
| C17H19N5O2 | |
| Molar mass | 325.365 g/mol |
| Appearance | Blue solid |
| Pharmacology | |
| L01DB11 (WHO) | |
| Intravenous | |
| Pharmacokinetics: | |
| 9.5–17.5 hours | |
| Fecal (main route of excretion) and renal (4–9%) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Pixantrone (rINN; trade name Pixuvri) is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue. It acts as a topoisomerase II poison and intercalating agent. The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.
Anthracyclines are important chemotherapy agents. However, their use is associated with irreversible and cumulative heart damage. Investigators have attempted to design related drugs that maintain the biological activity, but do not possess the cardiotoxicity of the anthracyclines. Pixantrone was developed to reduce heart damage related to treatment while retaining efficacy.
Random screening at the US National Cancer Institute of a vast number of compounds provided by the Allied Chemical Company led to the discovery of ametantrone as having significant anti-tumor activity. Further investigation regarding the rational development of analogs of ametantrone led to the synthesis of mitoxantrone, which also exhibited marked anti-tumor activity Mitoxantrone was considered as an analog of doxorubicin with less structural complexity but with a similar mode of action. In clinical studies, mitoxantrone was shown to be effective against numerous types of tumors with less toxic side effects than those resulting from doxorubicin therapy. However, mitoxantrone was not totally free of cardiotoxicity. A number of structurally modified analogs of mitoxantrone were synthesized and structure-activity relationship studies made. BBR 2778 was originally synthesized by University of Vermont researchers Miles P. Hacker and Paul A. Krapcho and initially characterized in vitro for tumor cell cytotoxicity and mechanism of action by studies at the Boehringer Mannheim Italia Research Center, Monza, and University of Vermont, Burlington. Other studies have been completed at the University of Texas M. D. Anderson Cancer Center, Houston, the Istituto Nazionale Tumori, Milan, and the University of Padua. In the search for novel heteroanalogs of anthracenediones, it was selected as the most promising compound. Toxicological studies indicated that BBR 2778 was not cardiotoxic, and US patents are held by the University of Vermont. An additional US patent application was completed in June 1995 by Boehringer Mannheim, Italy.
...
Wikipedia
