Phosphoenolpyruvate mutase
| phosphoenolpyruvate mutase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 5.4.2.9 | ||||||||
| CAS number | 115756-49-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
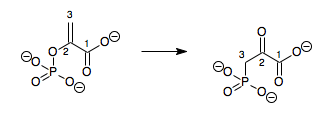
In enzymology, a phosphoenolpyruvate mutase (EC 5.4.2.9) is an enzyme that catalyzes the chemical reaction
Hence, this enzyme has one substrate, phosphoenolpyruvate (PEP), and one product, 3-phosphonopyruvate (PPR), which are structural isomers.
This enzyme belongs to the family of isomerases, specifically the phosphotransferases (phosphomutases), which transfer phosphate groups within a molecule. The systematic name of this enzyme class is phosphoenolpyruvate 2,3-phosphonomutase. Other names in common use include phosphoenolpyruvate-phosphonopyruvate phosphomutase, PEP phosphomutase, phosphoenolpyruvate phosphomutase, PEPPM, and PEP phosphomutase. This enzyme participates in aminophosphonate metabolism.
Phosphoenolpyruvate mutase was discovered in 1988.
As of late 2007, 6 structures have been solved for this class of enzymes, all by the Herzberg group [1] at the University of Maryland using PEPPM from the blue mussel, Mytilus edulis. The first structure (PDB accession code 1PYM) was solved in 1999 and featured a magnesium oxalate inhibitor. This structure identified the enzyme as consisting of identical beta barrel subunits (exhibiting the TIM barrel fold, which consists of eight parallel beta strands). Dimerization was observed in which a helix from each subunit interacts with the other subunit's barrel; the authors called this feature "helix swapping." The dimers can dimerize as well to form a homotetrameric enzyme. A double phosphoryl transfer mechanism was proposed on the basis of this study: this would involve breakage of PEP's phosphorus-oxygen bond to form a phosphoenzyme intermediate, followed by transfer of the phosphoryl group from the enzyme to carbon-3, forming PPR.
...
Wikipedia