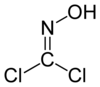
Phosgene oxime
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
dichloroformaldoxime
|
|||
| Other names
dichloroformoxime, CX
|
|||
| Identifiers | |||
|
1794-86-1 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
59024 |
||
| PubChem | 65582 | ||
|
|||
|
|||
| Properties | |||
| CHCl2NO | |||
| Molar mass | 113.93 g·mol−1 | ||
| Appearance | colorless crystalline solid or yellowish-brown liquid | ||
| Melting point | 35 to 40 °C (95 to 104 °F; 308 to 313 K) | ||
| Boiling point | 128 °C (262 °F; 401 K) | ||
| 70% | |||
| Hazards | |||
| Main hazards | highly toxic | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable odor and a violently irritating vapor.
Phosgene oxime can be prepared by reduction of chloropicrin:
The observation of a transient violet color in the reaction suggests intermediate formation of trichloronitrosomethane (Cl3CNO). Early preparations, using stannous chloride as the reductant, also started with chloropicrin.
The compound is electrophilic and thus sensitive to nucleophiles, including base hydrolysis:
Similar processes provide an easy way to destroy this dangerous compound. Hydrazine converts it to HCN and N2.
Phosgene oxime is classified as a vesicant even though it does not produce blisters. It is toxic by inhalation, ingestion, or skin contact. The effects of the poisoning occur almost immediately. No antidote for phosgene oxime poisoning is known. Generally, any treatment is supportive. Typical physical symptoms of CX exposure are as follows:
Phosgene oxime is highly soluble in water. It is corrosive to metals and also decomposes on contact with metals. It is rapidly hydrolysed by alkaline solutions. Adsorbent powders such as Fuller's earth or talcum powder can also be used.
...
Wikipedia