Phenothrin
 |
|
| Names | |
|---|---|
|
IUPAC name
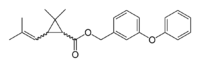
(3-Phenoxyphenyl)methyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate
|
|
| Other names
Sumithrin
Phenothrine Phenoxythrin Sumitrin Wellcide Pibutin Anvil Duet Anchimanaito 20S |
|
| Identifiers | |
|
26002-80-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:34916 |
| ChEMBL |
ChEMBL1322884 |
| ChemSpider |
4603 |
| ECHA InfoCard | 100.043.079 |
| EC Number | 247-404-5 |
| KEGG |
D08357 |
| MeSH | Phenothrin |
| PubChem | 4767 |
| UNII |
707484X33X |
|
|
|
|
| Properties | |
| C23H26O3 | |
| Molar mass | 350.451 g/mol |
| Melting point | <25 °C |
| Boiling point | >290 °C |
| Pharmacology | |
| P03AC03 (WHO) QP53AC03 (WHO) | |
| Hazards | |
| R-phrases | R36 R38 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Phenothrin, also called sumithrin and d-phenothrin, is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humans. d-Phenothrin is used as a component of aerosol insecticides for domestic use. Phenothrin is often used with methoprene, an insect growth regulator that interrupts the insect's biological life cycle by killing the eggs. Phenothrin is the active agent in the branded product Raid Fly & Wasp Killer.
Phenothrin is primarily used to kill fleas and ticks. It is also used to kill head lice in humans, but studies conducted in Paris, France and the United Kingdom have shown widespread resistance to phenothrin.
It is extremely toxic to bees. An U.S. Environmental Protection Agency (EPA) study found that 0.07 micrograms was enough to kill honey bees. It is also extremely toxic to aquatic life with a study showing concentrations of 0.03 ppb killing mysid shrimp. It has increased risk of liver cancer in rats and mice in long term exposure. It is capable of killing mosquitoes, although remains poisonous to cats and dogs, with seizures and deaths being reported due to poisoning. Specific data on concentrations or exposure is lacking.
Phenothrin has been found to possess antiandrogen properties, and was responsible for a small epidemic of gynecomastia via isolated environmental exposure.
The EPA has not assessed its effect on cancer in humans. However, one study performed by the Mt. Sinai School of Medicine links sumithrin with breast cancer; the link made by sumithrin's effect on increasing the expression of a gene responsible for mammary tissue proliferation.
In 2005, the U.S. EPA canceled permission to use phenothrin in several flea and tick products, at the request of the manufacturer, Hartz Mountain Industries. The products were linked to a range of adverse reactions, including hair loss, salivation, tremors, and numerous deaths in cats and kittens. In the short term, the agreement called for new warning labels on the products.
...
Wikipedia
