Phenibut
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Noofen |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 64–65% |
| Biological half-life | 5.3 hours (after 250 mg dose) |
| Identifiers | |
|
|
| Synonyms | Fenibut, Fenigam, Phenigam, Phenybut, Phenygam, Phenylgamma, PHG, PhGABA |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
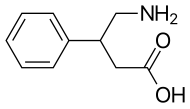
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol |
| 3D model (Jmol) | |
| Melting point | 253 °C (487 °F) |
|
|
|
|
|
|
|
Phenibut (fenibut, phenybut; brand names Noofen and Citrocard), contracted from β-phenyl-γ-aminobutyric acid (β-phenyl-GABA), is a central depressant and analog of the inhibitory neurotransmitter γ-aminobutyric acid (GABA), or a GABA analogue. The addition of a phenyl ring allows phenibut to cross the blood–brain barrier. Phenibut was developed in the Soviet Union in the 1960s, and has since been used there as a pharmaceutical drug to treat a wide range of ailments, including posttraumatic stress disorder, anxiety, depression, asthenia, insomnia, alcoholism, stuttering, and vestibular disorders, among other conditions. In some other parts of the world, phenibut is not approved for clinical use, and is instead sold as a nutritional supplement. It has been reported by some researchers to possess nootropic actions for its ability to improve neurological functions, but others have not observed these effects. It is generally accepted that phenibut has anxiolytic effects in both animal models and in humans.
Phenibut is a close structural analogue of GABA, as well as of baclofen (β-(4-chlorophenyl)-GABA), pregabalin (β-isobutyl-GABA), and GABOB (β-hydroxy-GABA). Phenibut is believed to act as a selective GABAB receptor agonist; studies are conflicting as to whether phenibut also acts as a GABAA receptor agonist. More recently, phenibut has been found to act preferentially as a blocker of α2δ subunit-containing voltage-gated calcium channels, similarly to gabapentin and pregabalin. As such, by definition, phenibut is a gabapentinoid.
...
Wikipedia
