Perifosine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
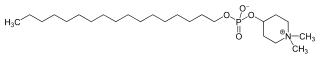
1,1-Dimethylpiperidinium-4-yl octadecyl phosphate
|
|
| Other names
D 21266; KRX 0401
|
|
| Identifiers | |
|
157716-52-4 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL372764 |
| ChemSpider | 130628 |
| ECHA InfoCard | 100.217.789 |
| 7424 | |
| PubChem | 148177 |
| UNII |
2GWV496552 |
|
|
|
|
| Properties | |
| C25H52NO4P | |
| Molar mass | 461.67 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Perifosine (also KRX-0401) is a drug candidate being developed for a variety of cancer indications. It is an alkyl-phospholipid structurally related to miltefosine. It acts as an Akt inhibitor and a PI3K inhibitor. It was being developed by Keryx Biopharmaceuticals who have licensed it from Æterna Zentaris Inc.
In 2010 perifosine has reached phase II. In one phase II trial for metastatic colon cancer perifosine doubled time to progression.
It has orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU.
In 2011 it was in a phase III trial for colorectal cancer, and another for multiple myeloma. On April 2, 2012, it was announced that perifosine failed its phase III clinical trial for treatment of colon cancer. Detailed results were released in June 2012. On March 11, 2013 Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma http://www.aezsinc.com/en/page.php?p=60&q=550.
...
Wikipedia
