Peridinin-chlorophyll binding protein
| Peridinin-chlorophyll A binding protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
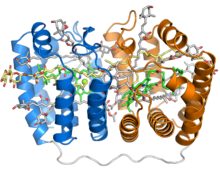
Crystal structure of the soluble peridinin-chlorophyll-protein complex from the photosynthetic dinoflagellate Amphidinium carterae. This complex is found in many photosynthetic dinoflagellates and involves a boat or cradle-shaped protein with two pseudosymmetrical repeats of eight alpha helices (shown in blue and orange) wrapped around a pigment-filled central cavity. Each eight-helix segment binds one chlorophyll molecule (green, with central magnesium ion shown as a green sphere), one diacylglycerol molecule (yellow) and four peridinin molecules (gray).
|
|||||||||
| Identifiers | |||||||||
| Symbol | PCP | ||||||||
| Pfam | PF02429 | ||||||||
| InterPro | IPR003376 | ||||||||
| SCOP | 1ppr | ||||||||
| SUPERFAMILY | 1ppr | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
The peridinin-chlorophyll-protein complex (PCP or PerCP) is a soluble molecular complex consisting of the peridinin-chlorophyll a-protein bound to peridinin, chlorophyll, and lipids. The peridinin molecules absorb light in the blue-green wavelengths (470 to 550 nm) and transfer energy to the chlorophyll molecules with extremely high efficiency. PCP complexes are found in many photosynthetic dinoflagellates, in which they may be the primary light-harvesting complexes.
The PCP protein has been identified in dinflagellate genomes in at least two forms, a homodimeric form composed of two 15-kD monomers, and a monomeric form of around 32kD believed to have evolved from the homodimeric form via gene duplication. The monomeric form consists of two pseudosymmetrical eight-helix domains in which the helices are packed in a complex topology resembling that of the beta sheets in a jelly roll fold. The three-dimensional arrangement of helices forms a boat-shaped molecule with a large central cavity in which the pigments and lipids are bound. Each eight-helix segment typically binds four peridinin molecules, one chlorophyll a molecule, and one lipid molecule such as digalactosyl diacyl glycerol; however, this stoichiometry varies among species and among PCP isoforms. The most common 4:1 peridinin:chlorophyll ratio was predicted by spectroscopy in the 1970s, but was unconfirmed until the crystal structure of the Amphidinium carterae PCP complex was solved in the 1990s. Whether formed from a protein monomer or dimer, the assembled protein-pigment complex is sometimes known as bPCP (for "building block") and is the minimal stable unit. In at least some PCP forms, including that from A. carterae, these building blocks assemble into a trimer thought to be the biologically functional state.
...
Wikipedia
