Palladium(II) acetate
 |
|||
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Palladium(II) acetate
|
|||
| Other names
Palladium diacetate
hexakis(acetato)tripalladium bis(acetato)palladium |
|||
| Identifiers | |||
|
3375-31-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
146827 |
||
| ECHA InfoCard | 100.020.151 | ||
| PubChem | 167845 | ||
| RTECS number | AJ1900000 | ||
|
|||
|
|||
| Properties | |||
| Pd(CH3COO)2 | |||
| Molar mass | 224.50 g/mol | ||
| Appearance | Brown yellow solid | ||
| Density | 2.19 g/cm3 | ||
| Melting point | 205 °C (401 °F; 478 K) decomposes | ||
| Boiling point | decomposes | ||
| low | |||
| Structure | |||
| monoclinic | |||
| Square Planar | |||
| 0 D | |||
| Hazards | |||
| Main hazards | considered nonhazardous | ||
| Safety data sheet | [1] | ||
| R-phrases | 41 | ||
| S-phrases | 24/25 | ||
| Related compounds | |||
|
Other anions
|
Palladium(II) chloride | ||
|
Other cations
|
Platinum(II) acetate | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Palladium(II) acetate is a chemical compound of palladium described by the formula Pd(O2CCH3)2 or Pd(OAc)2. It is considered more reactive than the analogous platinum compound. It is soluble in many organic solvents, and commonly used as a catalyst for organic reactions and as a precursor for the synthesis of other palladium catalysts.
Although the compound is a 1:2 stoichiometric ratio of palladium atoms and acetate ligands as their oxidation states suggest, the actual molecular nature of the chemical depends on how it is prepared.
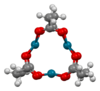
As prepared by Wilkinson and coworkers in 1965, and later studied by Skapski and Smart in 1970 by single crystal X-ray diffraction, palladium(II) acetate is a red-brown solid that crystallizes as monoclinic plates. Its structure was determined to be trimeric, consisting of an equilateral triangle of Pd atoms each pair of which is bridged with two acetate groups in a butterfly conformation. Each metal atom achieves approximate square planar co-ordination.
On the other hand, palladium(II) acetate prepared in a slightly different way was isolated as a pale pink powder, whose structure was determined by X-ray powder diffraction to consist of infinite chains of palladium atoms, each linked by an adjacent pair of acetate bridges to the next, in which the coordination geometry around each Pd is true square planar.
Palladium acetate, in trimeric form, can be prepared by refluxing palladium sponge with hot glacial acetic acid and nitric acid. An excess of palladium sponge metal or nitrogen gas flow is used to prevent contamination by a material that contains a nitrite ligand in place of one of the acetates (Pd3(OAc)5NO2).
The nitro variant has different solubility and catalytic activity in some reactions. Preventing, or controlling for the amount of, this impurity can be an important aspect for reliable use of palladium(II) acetate.
Palladium(II) propionate is prepared analogously; other carboxylates are prepared by reacting palladium(II) acetate with the appropriate carboxylic acid. Likewise, palladium(II) acetate can be prepared by reacting other palladium(II) carboxylates with acetic acid. This ligand exchange starting with a purified other carboxylate is an alternative way to synthesize palladium(II) acetate free from the nitro contaminant.
...
Wikipedia