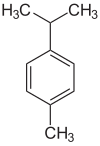
P-Cymene
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
1-Methyl-4-(propan-2-yl)benzene
|
|||
| Other names
p-Cymene (no longer recommended)
4-Isopropyltoluene 4-Methylcumene Paracymene |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.542 | ||
| EC Number | 202-796-7 | ||
| KEGG | |||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C10H14 | |||
| Molar mass | 134.21 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 0.857 g/cm3 | ||
| Melting point | −68 °C (−90 °F; 205 K) | ||
| Boiling point | 177 °C (351 °F; 450 K) | ||
| 23.4 mg/L | |||
| -102.8·10−6 cm3/mol | |||
| Hazards | |||
| R-phrases | R10 | ||
| S-phrases | S16 | ||
| Flash point | 47 °C (117 °F; 320 K) | ||
| 435 °C (815 °F; 708 K) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
p-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. There are two less common geometric isomers. o-Cymene, in which the alkyl groups are ortho-substituted, and m-cymene, in which they are meta-substituted. p-Cymene is the only natural isomer. All three isomers form the group of cymenes.
p-Cymene is insoluble in water, but miscible with ethanol and diethyl ether.
It is a constituent of a number of essential oils, most commonly the oil of cumin and thyme. Significant amounts are formed in sulfite pulping process from the wood terpenes.
p-Cymene is a common ligand for ruthenium. The parent compound is [(η6-cymene)RuCl2]2. This half-sandwich compound is prepared by the reaction of ruthenium trichloride with the terpene α-phellandrene. The osmium complex is also known.
...
Wikipedia