Olodaterol
 |
|
| Clinical data | |
|---|---|
| Trade names | Striverdi Respimat |
| AHFS/Drugs.com | UK Drug Information |
| Pregnancy category |
|
| Routes of administration |
Inhalation (MDI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~30% (inhalation) |
| Protein binding | ~60% |
| Metabolism | Hepatic |
| Biological half-life | 7.5 hours |
| Excretion | Feces (53%), urine (38%) — following IV administration |
| Identifiers | |
|
|
| Synonyms | BI 1744 CL |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
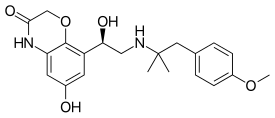
| Formula | C21H26N2O5 |
| Molar mass | 386.44 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Olodaterol (trade name Striverdi Respimat STRIH-ver-dee-RES-peh-mat) is an ultra-long-acting β adrenoreceptor agonist (ultra-LABA) used as an inhalation for treating patients with chronic obstructive pulmonary disease (COPD), manufactured by Boehringer Ingelheim.
Olodaterol is a once-daily maintenance bronchodilator treatment of airflow obstruction in patients with COPD including chronic bronchitis and/or emphysema, and is administered in an inhaler called Respimat Soft Mist Inhaler.
As of December 2013[update], olodaterol is not approved for the treatment of asthma. Olodaterol monotherapy was previously evaluated in four Phase II studies in asthma patients. However, currently there are no Phase III studies planned for olodaterol monotherapy in patients with asthma.
Adverse effects generally were rare and mild in clinical studies. Most common, but still affecting no more than 1% of patients, were nasopharyngitis (running nose), dizziness and rash. To judge from the drug's mechanism of action and from experiences with related drugs, hypertension (high blood pressure), tachycardia (fast heartbeat), hypokalemia (low blood levels of potassium), shaking, etc., might occur in some patients, but these effects have rarely, if at all, been observed in studies.
...
Wikipedia
