Nitisinone
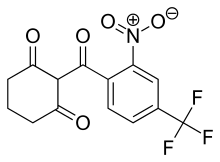 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | Approximately 54 h |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.218.521 |
| Chemical and physical data | |
| Formula | C14H10F3NO5 |
| Molar mass | 329.228 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Nitisinone (INN), also known as NTBC (an abbreviation of its full chemical name) is a medication used to slow the effects of hereditary tyrosinemia type 1. Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this rare condition. It is also being studied in the related condition alkaptonuria. It is marketed under the brand name Orfadin by the company Swedish Orphan Biovitrum (Sobi); it was first brought to market by Swedish Orphan International. It was originally developed as a candidate herbicide.
Nitisinone is used to treat hereditary tyrosinemia type 1, in combination with restriction of tyrosine in the diet.
Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this rare condition. I It is marketed under the brand name Orfadin.
It has been demonstrated that treatment with nitisinone can reduce urinary levels of homogentisic acid in alkaptonuria patients by 95%. A series of clinical trials run by DevelopAKUre to determine whether nitisinone is effective at treating the ochronosis suffered by patients with alkaptonuria are ongoing. If the trials are successful, DevelopAKUre will try to get nitisinone licensed for use by alkaptonuria patients.
The mechanism of action of nitisinone involves reversibile inhibition of 4-Hydroxyphenylpyruvate dioxygenase (HPPD),. This is a treatment for patients with Tyrosinemia type 1 as it prevents the formation of maleylacetoacetic acid and fumarylacetoacetic acid, which have the potential to be converted to succinyl acetone, a toxin that damages the liver and kidneys. This causes the symptoms of Tyrosinemia type 1 experienced by untreated patients.
...
Wikipedia
