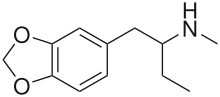
N-methyl-1,3-benzodioxol-5-yl)butan-2-amine
 |
|
Chemical structure
|
|
| Clinical data | |
|---|---|
| Synonyms | Methylbenzodioxolylbutanamine; N-Methyl-1,3-benzodioxolylbutanamine; MBDB; 3,4-Methylenedioxy-N-methyl-butanphenamine; MDMB |
| Legal status | |
| Legal status | |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.27 g/mol |
| 3D model (JSmol) | |
| Melting point | 156 °C (313 °F) |
|
|
|
|
1,3-Benzodioxolyl-N-methylbutanamine (N-methyl-1,3-benzodioxolylbutanamine, MBDB, 3,4-methylenedioxy-N-methyl-α-ethylphenylethylamine) is an entactogen of the phenethylamine chemical class. It is known by the street names Eden and Methyl-J. MBDB is a closely related chemical analogue of MDMA, with the only difference between the two molecules being an ethyl group instead of a methyl group attached to the alpha carbon. It has IC50 values of 784 nM against 5-HT, 7825 nM against dopamine, and 1233 nM against norepinephrine. Its metabolism has been described in scientific literature.
MBDB was first synthesized by pharmacologist and medicinal chemist David E. Nichols and later tested by Alexander Shulgin and described in his book, PiHKAL: A Chemical Love Story. MBDB's dosage, according to PiHKAL, is 180–210 mg; the proper dosage relative to body mass seems unknown. Its duration is 4–6 hours, with noticeable after-effects lasting for 1–3 hours.
MBDB was initially developed as a non-psychedelic entactogen. It has lower effects on the dopamine system in comparison to other entactogens such as MDMA. MBDB causes many mild, MDMA-like effects, such as lowering of social barriers and inhibitions, pronounced sense of empathy and compassion, mood lift, and mild euphoria are all present. MBDB tends to produce less euphoria, psychedelia, and stimulation in comparison.
...
Wikipedia
