Methyl Isocyanate
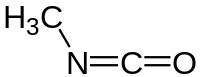 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Isocyanatomethane
|
|
| Other names
Methyl isocyanate
methyl carbylamine MIC |
|
| Identifiers | |
|
624-83-9 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:59059 |
| ChemSpider |
11727 |
| ECHA InfoCard | 100.009.879 |
| 6290 | |
| PubChem | 12228 |
|
|
|
|
| Properties | |
| H3CNCO | |
| Molar mass | 57.051 g/mol |
| Appearance | Colorless liquid |
| Odor | Sharp, pungent odor |
| Density | 0.9230 g/cm3 at 27 °C |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 38.3 °C (100.9 °F; 311.4 K) |
| 10% (15°C) | |
| Vapor pressure | 57.7 kPa |
| Structure | |
| 2.8 D | |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
−92.0 kJ·mol−1 |
| Hazards | |
|
EU classification (DSD)
|
|
| R-phrases | R12, R24/25, R26, R37/38, R41, R42/43, R63 |
| S-phrases | (S1/2), S26, S27/28, S36/37/39, S45, S63 |
| NFPA 704 | |
| Flash point | −7 °C (19 °F; 266 K) |
| 534 °C (993 °F; 807 K) | |
| Explosive limits | 5.3–26% |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
120 mg/kg (oral, mouse) 51.5 mg/kg (oral, rat) |
|
LC50 (median concentration)
|
6.1 ppm (rat, 6 hr) 12.2 ppm (mouse, 6 hr) 5.4 ppm (guinea pig, 6 hr) 21 ppm (rat, 2 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 0.02 ppm (0.05 mg/m3) [skin] |
|
REL (Recommended)
|
TWA 0.02 ppm (0.05 mg/m3) [skin] |
|
IDLH (Immediate danger)
|
3 ppm |
| Related compounds | |
|
Related compounds
|
Methyl isothiocyanate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane, methyl carbylamine, and MIC. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As a highly toxic and irritating material, it is extremely hazardous to human health. It was the principal toxicant involved in the Bhopal disaster, which killed nearly 2,259 people initially and officially 3,787 people in total.
Methyl isocyanate (MIC) is a colorless, lachrymatory (tearing agent), flammable liquid. It is soluble in water to 6–10 parts per 100 parts, but it also reacts with water (see Reactions below).
Methyl isocyanate is usually manufactured by the reaction of monomethylamine and phosgene. For large scale production, it is advantageous to combine these reactants at higher temperature in the gas phase. A mixture of methyl isocyanate and two moles of hydrogen chloride is formed, but N-methylcarbamoyl chloride (MCC) forms as the mixture is condensed, leaving one mole of hydrogen chloride as a gas.
The methyl isocyanate is obtained by treating the MCC with a tertiary amine, such as N,N-dimethylaniline, or with pyridine or by separating it by using distillation techniques.
Methyl isocyanate is also manufactured from N-methylformamide and air. In the latter process, it is immediately consumed in a closed-loop process to make methomyl. Other manufacturing methods have been reported.
...
Wikipedia

