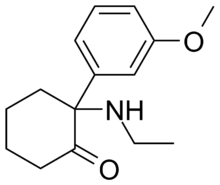
Methoxetamine
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 3-6 hours. |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.33 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Methoxetamine (MXE), or 3-MeO-2'-Oxo-PCE is a dissociative drug that has been sold as a designer drug. Methoxetamine differs from many dissociatives such as ketamine and phencyclidine that were developed as pharmaceuticals in that it was designed for grey market distribution.
Methoxetamine hydrochloride is soluble in ethanol up to 10 mg/ml at 25 °C.
Methoxetamine acts as a noncompetitive NMDA receptor antagonist, serotonin reuptake inhibitor, dopamine reuptake inhibitor, D2 receptor agonist, 5-HT2 receptor agonist, muscarinic acetylcholine receptor agonist, σ1 receptor agonist, μ receptor agonist, and κ receptor agonist.
The qualitative effects of methoxetamine were first described online in May 2010 and the compound became commercially available on a small scale in September 2010, by November use and sale of the methoxetamine had increased enough for it to be formally identified by the European Monitoring Centre for Drugs and Drug Addiction. By July 2011, the EMCDDA had identified 58 websites selling the compound at a cost of 145–195 euros for 10 grams. MXE remains popular despite bans in many countries.
Methoxetamine is reported to have a similar effect to ketamine, with increased potency and duration. Methoxetamine was often believed to possess opioid properties due to its structural similarity to 3-OH-PCP, but this assumption is not supported by data, which shows insignificant affinity for the µ-opioid receptor by the compound itself, but metabolites which form in-vivo might have differing effects. Recreational use of Methoxetamine has been associated with hospitalizations from high and/or combined consumption in the US and UK. Acute reversible cerebellar toxicity has been documented in three cases of hospital admission due to methoxetamine overdose, lasting for between one and four days after exposure.
Methoxetamine was designed in part to prevent the urotoxicity associated with ketamine abuse; it was thought the compound's increased potency and reduced dose would limit the accumulation of urotoxic metabolites in the bladder. Like ketamine, methoxetamine has been found to produce bladder inflammation and fibrosis after high dose, chronic administration in mice (although the dosages used were quite huge). Reports of urotoxicity in humans have yet to appear in the medical literature.
...
Wikipedia
