Meperidine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Demerol |
| Pregnancy category |
|
| Dependence liability |
High |
| Routes of administration |
oral, intravenous, intramuscular, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50–60% (Oral), 80-90% (Oral, in cases of hepatic impairment) |
| Protein binding | 65-75% |
| Metabolism | Liver |
| Biological half-life | 2.5-4 hours, 7-11 hours (liver disease) |
| Excretion | Renal |
| Identifiers | |
|
|
| Synonyms | meperidine |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.000.299 |
| Chemical and physical data | |
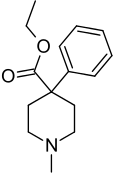
| Formula | C15H21NO2 |
| Molar mass | 247.33g/mol |
| 3D model (Jmol) | |
|
|
|
|
Pethidine, also known as meperidine and Demerol, is a synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1939 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany. Pethidine was the first wholly synthetic opioid developed and is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (pethidine, piminodine, anileridine and others), the prodines (alphaprodine, MPPP, etc.), bemidones (ketobemidone, etc.) and others more distant, including diphenoxylate and analogues.
Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as a hydrochloride salt in tablets, as a syrup, or by intramuscular, subcutaneous, or intravenous injection. For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1975, 60% of doctors prescribed it for acute pain and 22% for chronic severe pain.
Compared with morphine, pethidine was thought to be safer, carry a lower risk of addiction, and to be superior in treating the pain associated with biliary spasm or renal colic due to its putative anticholinergic effects. These were later discovered to be all myths, as it carries an equal risk of addiction, possesses no advantageous effects on biliary spasm or renal colic compared to other opioids, and due to its toxic metabolite norpethidine is more toxic than other opioids - especially during long-term use. The norpethidine metabolite was found to have serotonergic effects, so pethidine could, unlike most opioids, contribute to serotonin syndrome.
Pethidine is in Schedule II of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9230 with a 6250 kilo aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.87 for the hydrochloride and 0.84 for the hydrobromide. The A, B, and C intermediates in production of pethidine are also controlled, with ACSCN being 9232 for A (with a 6 gramme quota) and 9233 being B (quota of 11 grammes) and 9234 being C (6 gramme quota). It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as is morphine.
...
Wikipedia
