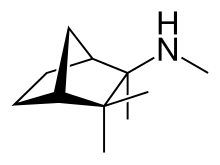
Mecamylamine
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 40% |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.000.433 |
| Chemical and physical data | |
| Formula | C11H21N |
| Molar mass | 167.291 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Mecamylamine (INN, BAN; or mecamylamine hydrochloride (USAN); brand names Inversine, Vecamyl) is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug. In the United States, it was withdrawn from the market for the treatment of hypertension in 2009.
Chemically, mecamylamine is a secondary aliphatic amine, with a pKaH of 11.2
Mecamylamine has been used as an orally-active ganglionic blocker in treating autonomic dysreflexia and hypertension, but, like most ganglionic blockers, it is more often used now as a research tool.
Mecamylamine is also sometimes used as an antiaddictive drug to help people stop smoking tobacco, and is now more widely used for this application than it is for lowering blood pressure. This effect is thought to be due to its blocking α3β4 nicotinic receptors in the brain. It has also been reported to bring about sustained relief from tics in Tourette syndrome when a series of more usually used agents had failed.
In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an augmentation therapy. This is the first substantive evidence that shows that compounds where the primary pharmacology is antagonism to neuronal nicotinic receptors will have antidepressant properties. TC-5214 is currently in Phase III of clinical development as an add-on treatment and on stage II as a monotherapy treatment for major depression. The first results reported from the Phase III trials showed that TC-5214 failed to meet the primary goal and the trial did not replicate the effects that had been encouraging in the Phase II trial. Development is funded by Targacept and AstraZeneca. It did not produce meaningful, beneficial results on patients as measured by changes on the Montgomery-Asberg Depression Rating Scale after eight weeks of treatment as compared with placebo.
...
Wikipedia
