Magic acid
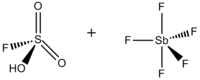 Fluorosulfuric acid-antimony pentafluoride 1:1
|
|
| Identifiers | |
|---|---|
|
23854-38-8 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
17339394 |
| ECHA InfoCard | 100.041.727 |
| PubChem | 16211378 |
|
|
|
|
| Properties | |
| HSbF6SO3 | |
| Molar mass | 316.82 g/mol |
| Appearance | Liquid |
| Hazards | |
| R-phrases | R14 R15/29 R16 R17 R18 R19 R26/27/28 R30 R31 R32 R33 R34 |
| S-phrases | S26 S27 S36/37/39 S38 S40 S41 S42 S43 S45 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted–Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.
The term "superacid" was first used in 1927 when James Bryant Conant found that perchloric acid could protonate ketones and aldehydes to form salts in nonaqueous solution. The term itself was coined by Gillespie later, after Conant combined sulfuric acid with fluorosulfuric acid, and found the solution to be several million times more acidic than sulfuric acid alone. The Magic Acid system was developed in the 1960s by George Olah, and was to be used to study stable carbocations. Gillespie also used the acid system to generate electron-deficient inorganic cations. The name originated after a Christmas party in 1966, when a member of the Olah lab placed a paraffin candle into the acid, and found that it dissolved quite rapidly. Examination of the solution with 1H-NMR showed a tert-butyl cation, suggesting that the paraffin chain that forms the wax had been cleaved, and then isomerized, the atoms were arranged into a different shape, to form the ion. The name appeared in a paper published by the Olah lab.
Although a 1:1 molar ratio of HSO3F and SbF5 best generates carbonium ions, the effects of the system at other molar ratios have also been documented. When the ratio SbF5:HSO3F is less than 0.2, the following two equilibria, determined by 19F NMR spectroscopy, are the most prominent in solution:
...
Wikipedia
