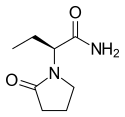
Levetiracetam
|
|||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /lɛvᵻtɪˈræsᵻtæm/ | ||
| Trade names | Keppra | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| License data |
|
||
| Pregnancy category |
|||
| Routes of administration |
Oral, intravenous | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | ~100% | ||
| Protein binding | <10% | ||
| Metabolism | Enzymatic hydrolysis of acetamide group | ||
| Biological half-life | 6–8 hrs | ||
| Excretion | Urinary | ||
| Identifiers | |||
|
|||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| ECHA InfoCard | 100.121.571 | ||
| Chemical and physical data | |||
| Formula | C8H14N2O2 | ||
| Molar mass | 170.209 g/mol | ||
| 3D model (Jmol) | |||
|
|||
|
|||
|
|
|||
Levetiracetam, marketed under the trade name Keppra among others, is a medication used to treat epilepsy. It is used for partial onset, myoclonic, or tonic-clonic seizures. It is the S-enantiomer of etiracetam.
Levetiracetam is available by mouth in two forms: immediate release and extended release. An immediate release tablet has been available as a generic in the United States since November 2008, and in the UK since 2011. The United States patent for the extended release tablet expires September 17, 2028. It was initially patented by UCB Pharma.
Levetiracetam has been approved in the United States as add-on treatment for partial (focal), myoclonic, and tonic-clonic seizures. Levetiracetam has been approved in the European Union as a monotherapy treatment for epilepsy in the case of partial seizures, or as an adjunctive therapy for partial, myoclonic, and tonic-clonic seizures. Levetiracetam has been shown to reduce partial (focal) seizures by 50% or more as an add-on medication. It is also used in veterinary medicine for similar purposes.
Levetiracetam is sometimes used off-label to treat status epilepticus or to prevent seizures associated with subarachnoid hemorrhages.
Levetiracetam has potential benefits for other psychiatric and neurologic conditions such as Tourette syndrome,anxiety disorder, and Alzheimer's disease. However, its most serious adverse effects are behavioral, and its benefit-risk ratio in these conditions is not well understood.
Levetiracetam has not been found to be useful for treatment of neuropathic pain, nor for treatment of essential tremors. Levetiracetam has not been found to be useful for treating autism, but is an effective treatment for partial, myoclonic, or tonic-clonic seizures associated with autism spectrum disorder.
...
Wikipedia