Leuprolide
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Lupron, Eligard, others |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a685040 |
| Pregnancy category |
|
| Routes of administration |
implant / injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 3 hours |
| Excretion | Kidney |
| Identifiers | |
|
|
| Synonyms | leuprolide (USAN) |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.161.466 |
| Chemical and physical data | |
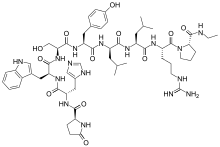
| Formula | C59H84N16O12 |
| Molar mass | 1209.4 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Leuprorelin, also known as leuprolide, is a manufactured version of a hormone used to treat prostate cancer, breast cancer, endometriosis, uterine fibroids, and early puberty. It is given by injection into a muscle or under the skin.
Common side effects include hot flashes, unstable mood, trouble sleeping, headaches, and pain at the site of injection. Other side effects may include high blood sugar, allergic reactions, and problems with the pituitary gland. Use during pregnancy may harm the baby. Leuprorelin is in the gonadotropin-releasing hormone (GnRH) analogue family of medication. It works by decreasing gonadotropin and therefore decreasing testosterone and estradiol.
Leuprorelin was approved for medical use in the United States in 1985. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United Kingdom a monthly dose costs the NHS about 75.24 pounds. In the United States the equivalent dose has a wholesale cost of 1,011.93 USD. It is sold under the brand name Lupron among others.
Leuprorelin may be used in the treatment of hormone-responsive cancers such as prostate cancer and breast cancer. It may also be used for estrogen-dependent conditions such as endometriosis or uterine fibroids.
...
Wikipedia
