Lanthanum trifluoride
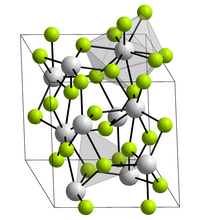 Crystal structure
|
|
| Names | |
|---|---|
| Other names
Lanthanum(III) fluoride
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.851 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| LaF3 | |
| Molar mass | 195.900 g/mol |
| Appearance | white, crystalline solid |
| Density | 5.9 g/cm3 |
| Melting point | 1,493 °C (2,719 °F; 1,766 K) |
| Structure | |
| Rhombohedral, hR24 | |
| P3c1, No. 165 | |
|
a = 0.7185 nm, c = 0.7351 nm
|
|
|
Lattice volume (V)
|
0.32865 |
|
Formula units (Z)
|
6 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine.
Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorides are close: three at the bottom corners of the trigonal prism, three in the faces of the trigonal prism, and three at top corners of the trigonal prism. There are also two fluorides a little further away above and below the prism. The cation can be considered 9-coordinate or 11-coordinate.
The larger sized rare earth elements (lanthanides), which are those with smaller atomic number, also form trifluorides with the LaF3 structure. Some actinides do as well.
The material is a component of multimetal fluoride glasses such as ZBLAN. It is also used (with europium trifluoride) in fluoride selective electrodes.
...
Wikipedia
