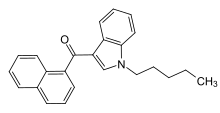
JWH-018
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Smoked, Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| ECHA InfoCard | 100.163.574 |
| Chemical and physical data | |
| Formula | C24H23NO |
| Molar mass | 341.45 g/mol |
| Solubility in water | hydrophobic, n/a mg/mL (20 °C) |
|
|
|
|
|
JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products such as "legal cannabis herbal incense blends" which in some countries are sold legally as "incense", labeled "not for human consumption".
John W. Huffman, an organic chemist at Clemson University, synthesized a variety of chemical compounds that affect the endocannabinoid system. JWH-018 is one of these compounds, with studies showing an affinity for the cannabinoid (CB1) receptor five times greater than that of THC. Cannabinoid receptors are found in mammalian brain and spleen tissue; however, the structural details of the active sites are currently unknown.
On December 15, 2008, it was reported by the German pharmaceutical company THC Pharm that JWH-018 was found as one of the active components in at least three versions of the herbal blend Spice, which has been sold as an incense in a number of countries around the world since 2002. An analysis of samples acquired four weeks after the German prohibition of JWH-018 took place found that the compound had been replaced with JWH-073.
JWH-018 is a full agonist of both the CB1 and CB2 cannabinoid receptors, with a reported binding affinity of 9.00 ± 5.00 nM at CB1 and 2.94 ± 2.65 nM at CB2. JWH-018 has an EC50 of 102 nM for human CB1 receptors, and 133 nM for human CB2 receptors. JWH-018 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, suggesting potent cannabinoid-like activity.
JWH-018 administered to rats resulted in the excretion of an indole-N-desalkyl metabolite as well as several hydroxylated metabolites in urine. The highest signals were observed for the hydroxylated N-desalkyl metabolites. Hydroxylation took place on the side chain and in both aromatic systems, the naphthalene and the indole rings, as could be shown by mass shift of the corresponding fragments and by MS3 experiments. Human metabolites were similar although most metabolism took place on the indole ring and pentyl side chain, and the hydroxylated metabolites were extensively conjugated with glucuronide.
...
Wikipedia
