Iron(II) bromide
 |
|
| Names | |
|---|---|
|
IUPAC name
Iron(II) bromide
|
|
| Other names
Ferrous bromide
|
|
| Identifiers | |
|
7789-46-0 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
74218 |
| ECHA InfoCard | 100.029.244 |
| PubChem | 659170 |
|
|
|
|
| Properties | |
| FeBr2 | |
| Molar mass | 215.65 g mol−1 |
| Appearance | yellow-brown solid |
| Density | 4.63 g cm−3, solid |
| Melting point | 684 °C (1,263 °F; 957 K) (anhydrous) 27 °C (Hexahydrate) |
| Boiling point | 934 °C (1,713 °F; 1,207 K) |
| soluble | |
| Solubility in other solvents | THF, methanol, ethanol |
| +13,600·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| Main hazards | none |
| R-phrases | R20 R36/37/38 |
| S-phrases | S26 S36 |
| Related compounds | |
|
Other anions
|
Iron(II) chloride |
|
Other cations
|
iron(III) bromide |
|
Related compounds
|
VBr2 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Iron(II) bromide is the chemical compound with the chemical formula FeBr2. The anhydrous compound is a yellow or brownish-colored paramagnetic solid. It is a common precursor to other iron compounds in research laboratory. Several hydrates of FeBr2 are also known.
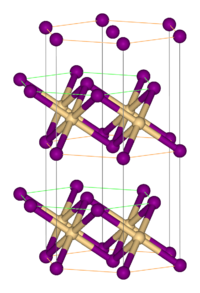
Like most metal halides, FeBr2 adopts a polymeric structure consisting of isolated metal centers cross-linked with halides. It crystallizes with the CdI2 structure, featuring close-packed layers of bromide ions, between which are located Fe(II) ions in octahedral holes. The packing of the halides is slightly different from that for FeCl2, which adopts the CdCl2 motif.
FeBr2 is synthesized using a methanol solution of concentrated hydrobromic acid and iron powder. gives the methanol solvate [Fe(MeOH)6]Br2 together with hydrogen gas. Heating the methanol complex in a vacuum gives pure FeBr2. Iron(II) bromide cannot be formed by the reaction of iron and bromine, because that reaction would produce ferric bromide.
FeBr2 reacts with 2 equivalents of (C2H5)4NBr to give [(C2H5)4N]2FeBr4.
FeBr2 reacts with bromide and bromine to form the intensely colored, mixed-valence species [FeBr3Br9]−.
FeBr2 is a weak reducing agent, as are all ferrous compounds.
...
Wikipedia
