Indium oxide
 |
|
| Names | |
|---|---|
| Other names
indium trioxide, indium sesquioxide
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.813 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| In2O3 | |
| Molar mass | 277.64 g/mol |
| Appearance | yellowish green odorless crystals |
| Density | 7.179 g/cm3 |
| Melting point | 1,910 °C (3,470 °F; 2,180 K) |
| insoluble | |
| Band gap | ~3 eV (300 K) |
| −56.0·10−6 cm3/mol | |
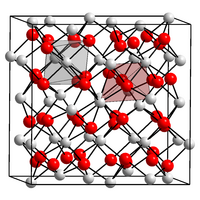
| Structure | |
| Cubic, space group Ia3 No. 206, cI80, a = 1.0117(1) nm, Z = 16 | |
| Hazards | |
|
EU classification (DSD)
|
not listed |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
Amorphous indium oxide is insoluble in water but soluble in acids, whereas crystalline indium oxide is insoluble in both water and acids. The crystalline form exist in two phases, the cubic (bixbyite type) and rhombohedral (corundum type). Both phases have a band gap of about 3 eV. The parameters of the cubic phase are listed in the infobox. The rhombohedral phase is produced at high temperatures and pressures or when using non-equilibrium growth methods. It has a space group R3c No. 167, Pearson symbol hR30, a = 0.5487 nm, b = 0.5487 nm, c = 0.57818 nm, Z = 6 and calculated density 7.31 g/cm3.
Thin films of chromium-doped indium oxide (In2−xCrxO3) are a magnetic semiconductor displaying high-temperature ferromagnetism, single-phase crystal structure, and semiconductor behavior with high concentration of charge carriers. It has possible applications in spintronics as a material for spin injectors.
Thin polycrystalline films of indium oxide doped with Zn are highly conductive (conductivity ~105 S/m) and even superconductive at helium temperatures. The superconducting transition temperature Tc depends on the doping and film structure and is below 3.3 K.
Bulk samples can be prepared by heating indium(III) hydroxide or the nitrate, carbonate or sulfate. Thin films of indium oxide can be prepared by sputtering of indium target in argon/oxygen atmosphere. They can be used as diffusion barriers ("barrier metals") in semiconductors, e.g. to inhibit diffusion between aluminium and silicon.
...
Wikipedia

