Hydroxides
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Hydroxide
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| OH− |
|||
| Molar mass | 17.01 g·mol−1 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
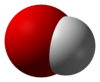
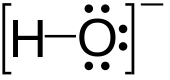
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.
The corresponding electrically neutral compound •HO is the hydroxyl radical. The corresponding covalently-bound group –OH of atoms is the hydroxyl group. Hydroxide ion and hydroxyl group are nucleophiles and can act as a catalyst in organic chemistry.
Many inorganic substances which bear the word "hydroxide" in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxyl groups.
...
Wikipedia