Hydralazine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Apresoline, BiDil, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682246 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26–50% |
| Protein binding | 85–90% |
| Metabolism | Liver |
| Onset of action | 5 to 30 min |
| Biological half-life | 2–8 hours, 7–16 hours (renal impairment) |
| Duration of action | 2 to 6 hrs |
| Excretion | Urine |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.001.528 |
| Chemical and physical data | |
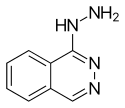
| Formula | C8H8N4 |
| Molar mass | 160.176 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Hydralazine, sold under the brand name Apresoline among others, is a medication used to treat high blood pressure and heart failure. This includes high blood pressure in pregnancy and very high blood pressure resulting in symptoms. It has been found to be particularly useful in heart failure together with isosorbide dinitrate in black people. It is given by mouth or by injection into a vein. Effects usually begin around 15 minutes and last up to six hours.
Common side effects include headache and fast heart rate. It is not recommended in people with coronary artery disease or rheumatic heart disease that is affecting the mitral valve. In those with kidney problems a low dose is recommended. Hydralazine is in the vasodilator family of medications and is believed to work by causing the dilation of blood vessels.
Hydralazine was discovered while scientists at Ciba were looking for a treatment for malaria. It was patented in 1949. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 2.78 to 9.11 USD per month. In the United States treatment costs about 50 to 100 USD per month.
...
Wikipedia
