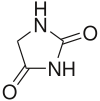
Hydantoins
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Imidazolidine-2,4-dione
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.650 | ||
| KEGG | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C3H4N2O2 | |||
| Molar mass | 100.08 g·mol−1 | ||
| Melting point | 220 °C (428 °F; 493 K) | ||
| 39.7 g/L (100 °C) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to a groups and a class of compounds with the same ring structure as the parent. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
Hydantoin was first isolated in 1861 by Adolf von Baeyer in the course of his study of uric acid. He obtained it by hydrogenation of allantoin, hence the name.
Friedrich Urech synthesized 5-methylhydantoin in 1873 from alanine sulfate and potassium cyanate in what is now known as the Urech hydantoin synthesis The method is very similar to the modern route using alkyl and arylcyanates. The 5,5-dimethyl compound can also be obtained from acetone cyanohydrin (also discovered by Urech: see cyanohydrin reaction) and ammonium carbonate. This reaction type is called the Bucherer–Bergs reaction.
According to the 1911 Encyclopædia Britannica, hydantoin can also be synthesized either by heating allantoin with hydroiodic acid or by "heating bromacetyl urea with alcoholic ammonia". The cyclic structure of hydantoins was confirmed by Dorothy Hahn 1913.
...
Wikipedia