Histrelin
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601146 |
| Pregnancy category |
|
| Routes of administration |
Subcutaneous implant |
| ATC code | L02AE05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 70% |
| Metabolism | Hepatic |
| Biological half-life | 4.0 hours |
| Excretion | Undetermined |
| Identifiers | |
|
|
| CAS Number |
76712-82-8 |
| PubChem (CID) | 25077993 |
| IUPHAR/BPS | 3884 |
| ChemSpider |
10482012 |
| UNII |
H50H3S3W74 |
| KEGG |
D02369 |
| ChEMBL |
CHEMBL1201255 |
| ECHA InfoCard | 100.163.860 |
| Chemical and physical data | |
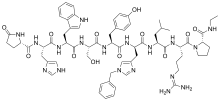
| Formula | C66H86N18O12 |
| Molar mass | 1323.5 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Histrelin acetate is a nonapeptide analog of gonadotropin-releasing hormone (GnRH) with added potency. When present in the bloodstream, it acts on particular cells of the pituitary gland called gonadotropes. Histrelin stimulates these cells to release luteinizing hormone and follicle-stimulating hormone. Thus it is considered a gonadotropin-releasing hormone agonist or GnRH agonist.
Histrelin is marketed by Endo Pharmaceuticals under the brand names Vantas and Supprelin LA.
Histrelin is used to treat hormone-sensitive cancers of the prostate in men and uterine fibroids in women. In addition, histrelin has been proven to be highly effective in treating central precocious puberty in children.
It is available as a daily intramuscular injection.
Histrelin is also available in a 12-month subcutaneous implant (Vantas) for the palliative treatment of advanced prostate cancer, since 2005 in the US, and since Jan 2010 in the UK.
A 12-month subcutaneous implant (Supprelin LA) for central precocious puberty (CPP) was approved on May 3, 2007 by the U.S. Food and Drug Administration.
Histrelin is also part of the primary care protocol in transgender children/youth, and is used in suppressing cis-sex puberty, until the patient is ready to begin cross-sex hormonal therapy.
Side effects are mainly due to low testosterone levels and include headache, hot flashes, reduced libido, and erectile dysfunction.
In a process known as downregulation, daily stimulation of pituitary gonadotropes causes them to become desensitized to the effects of histrelin. As a consequence, levels of LH and FSH fall after a short period of time. From that point forward, as long as histrelin is administered, the levels of LH and FSH in the blood remain low.
...
Wikipedia
