Histidine phosphotransfer protein
| Histidine phosphotransfer domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
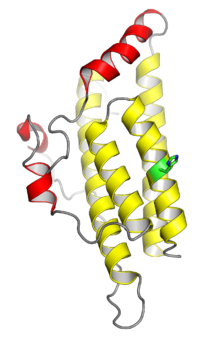
The crystal structure of the yeast histidine phosphotransferase protein Ypd1. The four helices shown in yellow comprise the conserved four-helix bundle typical of monomeric HPt domains; the helices shown in red are insertions specific to Ypd1. The histidine phosphorylation site is shown in green. From PDB: 1C02.
|
|||||||||
| Identifiers | |||||||||
| Symbol | Hpt | ||||||||
| Pfam | PF01627 | ||||||||
| InterPro | IPR008207 | ||||||||
| SMART | HPT | ||||||||
| PROSITE | PS50894 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| Histidine phosphotransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|

The crystal structure of the Caulobacter crescentus histidine phosphotransferase protein ChpT in dimeric form. The four helices shown in yellow comprise the conserved four-helix bundle, with the histidine phosphorylation sites highlighted in green. The domains shown in red and tan are pseudo-CA domains that resemble the ATP-binding domains of histidine kinases, but do not bind or hydrolyze ATP. From PDB: 4FMT.
|
|||||||||
| Identifiers | |||||||||
| Symbol | HPTransfase | ||||||||
| Pfam | PF10090 | ||||||||
| InterPro | IPR018762 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Histidine phosphotransfer domains and histidine phosphotransferases (both often abbreviated HPt) are protein domains involved in the "phosphorelay" form of two-component regulatory systems. These proteins possess a phosphorylatable histidine residue and are responsible for transferring a phosphoryl group from an aspartate residue on an intermediate "receiver" domain, typically part of a hybrid histidine kinase, to an aspartate on a final response regulator.
In orthodox two-component signaling, a histidine kinase protein autophosphorylates on a histidine residue in response to an extracellular signal, and the phosphoryl group is subsequently transferred to an aspartate protein on the receiver domain of a response regulator. In phosphorelays, the "hybrid" histidine kinase contains an internal aspartate-containing receiver domain to which the phosphoryl group is transferred, after which an HPt protein containing a phosphorylatable histidine receives the phosphoryl group and finally transfers it to the response regulator. The relay system thus progresses in the order His-Asp-His-Asp, with the second His contributed by Hpt. In some cases, a phosphorelay system is constructed from four separate proteins rather than a hybrid histidine kinase with an internal receiver domain, and in other examples both the receiver and the HPt domains are present in the histidine kinase polypeptide chain. A census of two-component system domain architecture found that HPt domains in bacteria are more common as domains of larger proteins than they are as individual proteins.
The increased complexity of the phosphorelay system compared to orthodox two-component signaling provides additional opportunities for regulation and improves the specificity of the response. Although there is very little cross-talk between orthodox two-component systems, phosphorelays allow more complex signaling pathways; examples include a bifurcated pathway with multiple downstream outputs, as in the case of the Caulobacter crescentus ChpT HPt involved in cell cycle regulation, or, alternatively, pathways in which more than one histidine kinase controls a single response regulator, such as the sporulation pathway in Bacillus subtilis, which can give rise to complex temporal variations. In some known cases, there is an additional form of regulation in phosphohistidine phosphatase enzymes that act on HPt, such as the Escherichia coli protein SixA which targets ArcB.
...
Wikipedia
