Hexon protein
| Adeno_hexon | |||||||||
|---|---|---|---|---|---|---|---|---|---|
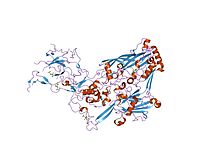
refinement of adenovirus type 2 hexon with cns
|
|||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon | ||||||||
| Pfam | PF01065 | ||||||||
| InterPro | IPR016107 | ||||||||
| SCOP | 1dhx | ||||||||
| SUPERFAMILY | 1dhx | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| Adeno_hexon_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|

the quasi-atomic model of human adenovirus type 5 capsid (part 2)
|
|||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon_C | ||||||||
| Pfam | PF03678 | ||||||||
| InterPro | IPR016108 | ||||||||
| SCOP | 1dhx | ||||||||
| SUPERFAMILY | 1dhx | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
In molecular biology, the hexon protein is a major coat protein found in Adenoviruses. Hexon coat proteins are synthesised during late infection and form homo-trimers. The 240 copies of the hexon trimer that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of polypeptide IX. The penton complex, formed by the peripentonal hexons and base hexon (holding in place a fibre), lie at each of the 12 vertices. The hexon coat protein is a duplication consisting of two domains with a similar fold packed together like the nucleoplasmin subunits. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The domains have a beta-sandwich structure consisting of 8 strands in two sheets with a jelly-roll topology; each domain is heavily decorated with many insertions. Some hexon proteins contain a distinct C-terminal domain.
In a recent paper (Bremner et al., 2009) it was shown that hexon directly recruits the cellular motor protein dynein in a pH-dependent manner. The dynein-regulatory protein, dynactin, was found to play a clear role in regulating the dynein-adenovirus complex transport to the nucleus.
This article incorporates text from the public domain Pfam and InterPro IPR016107
...
Wikipedia
