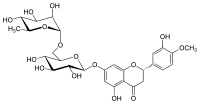
Hesperidin
 |
|
| Names | |
|---|---|
|
IUPAC name
(2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one
|
|
| Other names
Hesperetin 7-rutinoside
|
|
| Identifiers | |
|
520-26-3 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:28775 |
| ChEMBL |
ChEMBL449317 |
| ChemSpider |
10176 |
| ECHA InfoCard | 100.007.536 |
| PubChem | 10621 |
| UNII |
E750O06Y6O |
|
|
|
|
| Properties | |
| C28H34O15 | |
| Molar mass | 610.57 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Hesperidin is a flavan-on glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist Lebreton from the white inner layer of citrus peels (mesocarp, albedo).
Hesperidin is believed to play a role in plant defense.
Peppermint also contains hesperidin.
Hesperidin 6-O-alpha-L-rhamnosyl-beta-D-glucosidase, an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose, is found in the Ascomycetes species.
As a flavanone found in citrus fruits (such as oranges, lemons or pummelo fruits), hesperidin is under laboratory research for possible biological properties. One area of research is focused on the possible chemopreventive effects of hesperidin, but there is no current proof that hesperidin has this role in human cancer mechanisms.
...
Wikipedia
