Glycerine
 |
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Propane-1,2,3-triol
|
|||
| Other names
Glycerin
Glycerine Propanetriol 1,2,3-Trihydroxypropane 1,2,3-Propanetriol |
|||
| Identifiers | |||
|
56-81-5 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:17522 |
||
| ChEMBL |
ChEMBL692 |
||
| ChemSpider |
733 |
||
| DrugBank |
DB04077 |
||
| ECHA InfoCard | 100.000.263 | ||
| E number | E422 (thickeners, ...) | ||
| 5195 | |||
| KEGG |
D00028 |
||
| PubChem | 753 | ||
| UNII |
PDC6A3C0OX |
||
|
|||
|
|||
| Properties | |||
| C3H8O3 | |||
| Molar mass | 92.09 g·mol−1 | ||
| Appearance | colorless liquid hygroscopic |
||
| Odor | odorless | ||
| Density | 1.261 g/cm3 | ||
| Melting point | 17.8 °C (64.0 °F; 290.9 K) | ||
| Boiling point | 290 °C (554 °F; 563 K) | ||
| miscible | |||
| Vapor pressure | 0.003 mmHg (50°C) | ||
| -57.06·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.4746 | ||
| Viscosity | 1.412 Pa·s | ||
| Pharmacology | |||
| A06AG04 (WHO) A06AX01 (WHO), QA16QA03 (WHO) | |||
| Hazards | |||
| Safety data sheet |
See: data page JT Baker |
||
| NFPA 704 | |||
| Flash point | 160 °C (320 °F; 433 K) (closed cup) 176 °C (349 °F; 449 K) (open cup) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp) | ||
|
REL (Recommended)
|
None established | ||
|
IDLH (Immediate danger)
|
N.D. | ||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
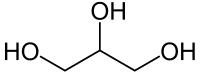
Glycerol /ˈɡlɪsərɒl/ (also called glycerine or glycerin; see spelling differences) is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in all lipids known as triglycerides. It is widely used in the food industry as a sweetener and humectant and in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature.
Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels each carbon as either sn-1, sn-2, or sn-3.
Glycerol is generally obtained from plant and animal sources where it occurs as triglycerides. Triglycerides are esters of glycerol with long-chain carboxylic acids. The hydrolysis, saponification, or transesterification of these triglycerides produces glycerol as well as the fatty acid derivative:
Triglycerides (1) are treated with an alcohol such as ethanol (2) with catalytic base to give ethyl esters of fatty acids (3) and glycerol (4):
...
Wikipedia