Glucose-6-phosphate dehydrogenase
| Glucose-6-phosphate dehydrogenase, NAD binding domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
glucose 6-phosphate dehydrogenase from leuconostoc mesenteroides
|
|||||||||
| Identifiers | |||||||||
| Symbol | G6PD_N | ||||||||
| Pfam | PF00479 | ||||||||
| Pfam clan | CL0063 | ||||||||
| InterPro | IPR022674 | ||||||||
| PROSITE | PDOC00067 | ||||||||
| SCOP | 1dpg | ||||||||
| SUPERFAMILY | 1dpg | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| Glucose-6-phosphate dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.1.1.49 | ||||||||
| CAS number | 9001-40-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) (EC 1.1.1.49) is a cytosolic enzyme that catalyzes the chemical reaction
This enzyme participates in the pentose phosphate pathway (see image), a metabolic pathway that supplies reducing energy to cells (such as erythrocytes) by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate (NADPH). The NADPH in turn maintains the level of glutathione in these cells that helps protect the red blood cells against oxidative damage from compounds like hydrogen peroxide. Of greater quantitative importance is the production of NADPH for tissues actively engaged in biosynthesis of fatty acids and/or isoprenoids, such as the liver, mammary glands, adipose tissue, and the adrenal glands. G6PD reduces NADP+ to NADPH while oxidizing glucose-6-phosphate.
Clinically, an X-linked genetic deficiency of G6PD predisposes a person to non-immune hemolytic anemia .
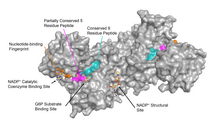
G6PD is widely distributed in many species from bacteria to humans. Multiple sequence alignment of over 100 known G6PDs from different organisms reveal sequence identity ranging from 30% to 94%. Human G6PD has over 30% identity in amino acid sequence to G6PD sequences from other species. Humans also have two isoforms of a single gene coding for G6PD. Moreover, 150 different human G6PD mutants have been documented. These mutations are mainly missense mutations that result in amino acid substitutions, and while some of them result in G6PD deficiency, others do not seem to result in any noticeable functional differences. Some scientists have proposed that some of the genetic variation in human G6PD resulted from generations of adaptation to malarial infection.
...
Wikipedia
