Fosfomycin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Monurol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697008 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | J01XX01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30–37% (oral, fosfomycin tromethamine); varies with food intake |
| Protein binding | Nil |
| Metabolism | Nil |
| Biological half-life | 5.7 hours (mean) |
| Excretion | Renal and fecal, unchanged |
| Identifiers | |
|
|
| CAS Number |
23155-02-4 |
| PubChem (CID) | 446987 |
| DrugBank |
DB00828 |
| ChemSpider |
394204 |
| UNII |
2N81MY12TE |
| KEGG |
D04253 |
| ChEBI |
CHEBI:28915 |
| ChEMBL |
CHEMBL1757 |
| ECHA InfoCard | 100.041.315 |
| Chemical and physical data | |
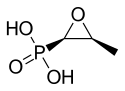
| Formula | C3H7O4P |
| Molar mass | 138.059 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 94 °C (201 °F) |
|
|
|
|
Fosfomycin (also known as phosphomycin, phosphonomycin and the trade name Monurol and Monuril) is a broad-spectrum antibiotic produced by certain Streptomyces species, although it can now be made by chemical synthesis.
As a single-dose, fosfomycin is more convenient than a multiple-dose therapy norfloxacin, for the same anti-bacterial efficacy.
Fosfomycin (originally known as phosphonomycin) was discovered in a joint effort of Merck and Co. and Spain's Compañía Española de Penicilina y Antibióticos (CEPA). It was first isolated by screening broth cultures of Streptomyces fradiae isolated from soil samples for the ability to cause formation of spheroplasts by growing bacteria. The discovery was described in a series of papers published in 1969. CEPA began producing fosfomycin on an industrial scale in 1971 at its Aranjuez facility.
Fosfomycin is indicated in the treatment of urinary tract infections, where it is usually administered as a single oral megadose. Its use in combination with tobramycin to treat lung infections in patients with cystic fibrosis was also explored.
The drug is well tolerated and has a low incidence of harmful side-effects. However, development of bacterial resistance under therapy is a frequent occurrence and makes fosfomycin unsuitable for sustained therapy of severe infections. It is not recommended for children and those over 75 years old.
Additional uses have been proposed. The global problem of advancing antimicrobial resistance has led to a renewed interest in its use more recently.
Fosfomycin is bactericidal and inhibits bacterial cell wall biogenesis by inactivating the enzyme UDP-N-acetylglucosamine-3-enolpyruvyltransferase, also known as MurA. This enzyme catalyzes the committed step in peptidoglycan biosynthesis, namely the ligation of phosphoenolpyruvate (PEP) to the 3'-hydroxyl group of UDP-N-acetylglucosamine. This pyruvate moiety provides the linker that bridges the glycan and peptide portion of peptidoglycan. Fosfomycin is a PEP analog that inhibits MurA by alkylating an active site cysteine residue (Cys 115 in the Escherichia coli enzyme).
...
Wikipedia
