Fosamax
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Fosamax |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601011 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0.6% |
| Metabolism | excreted unchanged |
| Biological half-life | 126 months |
| Excretion | renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.128.415 |
| Chemical and physical data | |
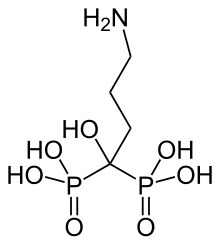
| Formula | C4H13NO7P2 |
| Molar mass | 249.097 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Alendronic acid (INN) or alendronate sodium (USAN) — sold as Fosamax by Merck — is a bisphosphonate drug used for osteoporosis, osteogenesis imperfecta, and several other bone diseases. It is marketed alone as well as in combination with vitamin D (2,800 IU and 5,600 IU, under the name Fosamax+D). Merck's U.S. patent on alendronate expired in 2008 and the drug is now available as a generic. This is the most widely prescribed bisphosphonate medicine in the United States .
As with all potent bisphosphonates, the fraction of the drug that reaches the circulatory system intact (systemic bioavailability) after oral dosing is low, averaging only 0.6–0.7% in women and in men under fasting conditions. Intake together with meals and beverages other than water further reduces the bioavailability. The absorbed drug rapidly partitions, with approximately 50% binding to the exposed bone surface; the remainder is excreted unchanged by the kidneys. Unlike with most drugs, the strong negative charge on the two phosphonate moieties limits oral bioavailability, and, in turn, the exposure to tissues other than bone is very low. After absorption in the bone, alendronate has an estimated terminal elimination half-life of 10 years.
Alendronate inhibits osteoclast-mediated bone-resorption. Like all bisphosphonates, it is chemically related to inorganic pyrophosphate, the endogenous regulator of bone turnover. But while pyrophosphate inhibits both osteoclastic bone resorption and the mineralization of the bone newly formed by osteoblasts, alendronate specifically inhibits bone resorption without any effect on mineralization at pharmacologically achievable doses. Its inhibition of bone-resorption is dose-dependent and approximately 1,000 times stronger than the equimolar effect of the first bisphosphonate drug, etidronate. Under therapy, normal bone tissue develops, and alendronate is deposited in the bone-matrix in a pharmacologically inactive form. For optimal action, enough calcium and vitamin D are needed in the body in order to promote normal bone development. Hypocalcemia should, therefore, be corrected before starting therapy.
...
Wikipedia
