Forodesine
 |
|
| Names | |
|---|---|
|
IUPAC name
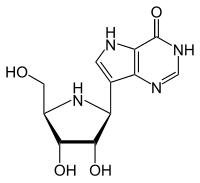
7-[(2S,3S,4R,5R)-3,4-Dihydroxy-5-(hydroxymethyl)-2-pyrrolidinyl]-1,5-dihydropyrrolo[2,3-e]pyrimidin-4-one
|
|
| Other names
Immucillin H
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C11H14N4O4 | |
| Molar mass | 266.26 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Forodesine (INN; also known as Immucillin H) is a transition-state analog inhibitor of purine nucleoside phosphorylase studied for the treatment of patients with T-cell acute lymphoblastic leukemia (T-ALL) and for treatment of B-cell acute lymphocytic leukemia (B-ALL).
Forodesine was originally discovered by Vern Schramm's laboratory at the Albert Einstein College of Medicine in New York and Industrial Research Limited in New Zealand.
Forodesine is being developed by BioCryst Pharmaceuticals as Fodosine. As of 2008[update], it is currently in phase II clinical trials..
In 2006, BioCryst announced an licensing agreement with Mundipharma International Holdings Limited (“Mundipharma”) to develop and commercialize Fodosine in markets across Europe, Asia, and Australasia for use in oncology.
...
Wikipedia
