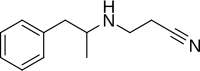
Fenproporex
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | partly converted to amphetamine (30 to 60%) |
| Excretion | urine, mainly as amphetamine, about 5 to 9% unchanged |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.036.752 |
| Chemical and physical data | |
| Formula | C12H16N2 |
| Molar mass | 188.269 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Fenproporex (Perphoxene) is a stimulant drug of the phenethylamine and amphetamine chemical classes which was developed in the 1960s. It is used as an appetite suppressant for the treatment of obesity.
Fenproporex produces amphetamine as a metabolite, and was withdrawn in many countries following problems with abuse, but it is still prescribed in some countries. It is sometimes combined with benzodiazepines, antidepressants and other compounds to create the "Brazilian diet pill".
Fenproporex has never been approved by the US Food and Drug Administration (FDA) for sale in the US due to lack of efficacy and safety data. However, in March 2009 the FDA warned consumers that it has been detected as an unlabeled component of diet pills available over the Internet. Fenproporex is designated a Schedule IV controlled substance in the US pursuant to the Controlled Substances Act.
Fenproporex is on the list of substances banned by the World Anti-Doping Agency, and any sportsperson testing positive for the substance faces a ban from competition.
...
Wikipedia
