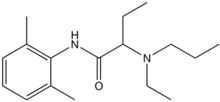
Etidocaine
 |
|
| Clinical data | |
|---|---|
| Trade names | Duranest |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a603026 |
| Pregnancy category |
|
| Routes of administration |
Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | Hepatic |
| Biological half-life | 2.5 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.048.296 |
| Chemical and physical data | |
| Formula | C17H28N2O |
| Molar mass | 276.42 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Etidocaine, marketed under the trade name Duranest, is a local anesthetic given by injection during surgical procedures and labor and delivery. Etidocaine has a long duration of activity, and the main disadvantage of using during dentistry is increased bleeding during surgery.
...
Wikipedia
