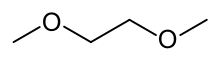
Ethylene glycol dimethyl ether
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
1,2-Dimethoxyethane
|
|
| Other names
Ethane-1,2-diyl dimethyl ether
DME Glyme Ethylene glycol dimethyl ether Monoglyme Dimethyl glycol Dimethyl cellosolve |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.451 |
| RTECS number | KI1451000 |
|
|
|
|
| Properties | |
| C4H10O2 | |
| Molar mass | 90.12 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8683 g/cm3 |
| Melting point | −58 °C (−72 °F; 215 K) |
| Boiling point | 85 °C (185 °F; 358 K) |
| miscible | |
| Hazards | |
|
EU classification (DSD) (outdated)
|
Flammable (F) Toxic (T) Repr. Cat. 2 |
| R-phrases (outdated) | R60, R61, R11, R19, R20 |
| S-phrases (outdated) | S53, S45 |
| NFPA 704 | |
| Flash point | −2 °C (28 °F; 271 K) |
| Related compounds | |
|
Related Ethers
|
Dimethoxymethane |
|
Related compounds
|
Ethylene glycol 1,4-Dioxane Diethylene glycol dimethyl ether |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water.
Monoglyme can in principle be produced by a number of methods. In practice it is produced industrially by the reaction of dimethylether with ethylene oxide. Other methods include:
Together with a high-permittivity solvent (e.g. propylene carbonate), dimethoxyethane is used as the low-viscosity component of the solvent for electrolytes of lithium batteries. In the laboratory, DME is used as a coordinating solvent.
Dimethoxyethane is often used as a higher boiling alternative to diethyl ether and THF. Dimethoxyethane forms chelate complexes with cations and acts as a bidentate ligand. It is therefore often used in organometallic chemistry like Grignard reactions, hydride reductions, and palladium-catalyzed reactions like Suzuki reactions and Stille couplings. Dimethoxyethane is also a good solvent for oligo- and polysaccharides.
...
Wikipedia

