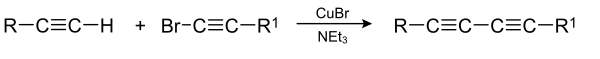Eglinton coupling
| Cadiot–Chodkiewicz coupling | |
|---|---|
| Named after | Paul Cadiot Wladyslav Chodkiewicz |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | cadiot-chodkiewicz-coupling |
| RSC ontology ID | RXNO:0000100 |
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base. The reaction product is a 1,3-diyne or di-alkyne.
The reaction mechanism involves deprotonation by base of the terminal alkyne proton followed by formation of a copper(I) acetylide. A cycle of oxidative addition and reductive elimination on the copper centre then creates a new carbon-carbon bond.
Unlike the related Glaser coupling the Cadiot–Chodkiewicz coupling proceeds selectively and will only couple the alkyne to the haloalkyne, giving a single product. By compassison the Glaser coupling would simply produce a distribution of all possible couplings. In one study the Cadiot–Chodkiewicz coupling has been applied in the synthesis of acetylene macrocycles starting from cis-1,4-diethynyl-1,4-dimethoxycyclohexa-2,5-diene. This compound is also the starting material for the dibromide through N-bromosuccinimide (NBS) and silver nitrate:
...
Wikipedia