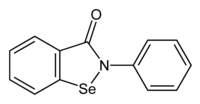
Ebselen
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
2-Phenyl-1,2-benzoselenazol-3-one
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.132.190 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C13H9NOSe | |
| Molar mass | 274.17666 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ebselen (also called PZ 51, DR3305, and SPI-1005), is a synthetic organoselenium drug molecule with anti-inflammatory, anti-oxidant and cytoprotective activity. It acts as a mimic of glutathione peroxidase and can also react with peroxynitrite. It is being investigated as a possible treatment for reperfusion injury and stroke,hearing loss and tinnitus, and bipolar disorder.
Additionally, ebselen may be effective against Clostridium difficile infections.
Ebselen is a potent scavenger of hydrogen peroxide as well as hydroperoxides including membrane bound phospholipid and cholesterylester hydroperoxides. Several ebselen analogs have been shown to scavenge hydrogen peroxide in the presence of thiols.
Generally, synthesis of the characteristic scaffold of ebselen, the benzoisoselenazolone ring system, can be achieved either through reaction of primary amines (RNH2) with 2-(chloroseleno)benzoyl chloride (Route I), by ortho-lithiation of benzanilides followed by oxidative cyclization (Route II) mediated by cupric bromide (CuBr2), or through the efficient Cu-catalyzed selenation / heterocyclization of o-halobenzamides, a methodology developed by Kumar et al. (Route III).
...
Wikipedia

