Drug-eluting stents
| Drug-eluting stent | |
|---|---|
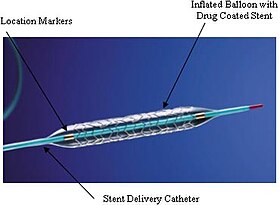
An example of a drug-eluting stent. This is the TAXUS Express2 Paclitaxel-Eluting Coronary Stent System, which releases paclitaxel.
|
|
| ICD-9-CM | 00.55 |
| MeSH | D054855 |
A drug-eluting stent (DES) is a peripheral or coronary stent (a scaffold) placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (thrombi), could otherwise block the stented artery, a process called restenosis. The stent is usually placed within the peripheral or coronary artery by an interventional cardiologist or interventional radiologist during an angioplasty procedure.
Drug-eluting stents in current clinical use were approved by the FDA after clinical trials showed they were statistically superior to bare-metal stents for the treatment of native coronary artery narrowings, having lower rates of major adverse cardiac events (usually defined as a composite clinical endpoint of death + myocardial infarction + repeat intervention because of restenosis). The first drug-eluting stents to be approved in Europe and the U.S. were coated with paclitaxel or an mTOR inhibitor, such as sirolimus.
Clinical trials have shown the benefits of coronary stenting with bare-metal stents over other methods of angioplasty, including balloon angioplasty and atherectomy. Drug-eluting stents (DES) have also been extensively studied, and are generally superior to bare-metal stents with respect to occurrence of major adverse cardiac events (MACE, generally defined as death, myocardial infarction, or the need for a repeat revascularization procedure). Stents are indicated to improve the diameter of the coronary artery lumen, when narrowing (generally because of atherosclerosis) causes ischemia (reduced oxygen delivery to the muscle supplied by that artery).
...
Wikipedia
