Disulfur dibromide
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
bromosulfanyl thiohypobromite
|
|||
| Other names
Sulphur dibromide
Sulfur(II) bromide Dibromosulfane Bromosulfanyl thiohypobromite |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ECHA InfoCard | 100.032.821 | ||
| EC Number | 236-119-1 | ||
|
PubChem CID
|
|||
|
|||
| Properties | |||
| Br2S2 | |||
| Molar mass | 223.940 g mol−1 | ||
| Appearance | orange/yellow liquid | ||
| Density | 2.703 | ||
| Boiling point | 54 °C (129 °F; 327 K) | ||
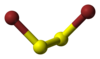
| Structure | |||
| C2, gauche | |||
| Hazards | |||
| Safety data sheet | ICSC 1661 | ||
|
EU classification (DSD) (outdated)
|
Corrosive (C) Irritant (Xi) Dangerous for the environment (N) |
||
| R-phrases (outdated) | R14, R34, R37, R50 | ||
| S-phrases (outdated) | (S1/2), S26, S45, S61 | ||
| Related compounds | |||
|
Related
|
Sulfur dibromide Thionyl bromide Sulfuryl bromide |
||
|
Related compounds
|
Disulfur difluoride Disulfur dichloride |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Disulfur dibromide is the chemical compound whose molecule is composed of two atoms each of sulfur and bromine. The molecular structure is akin to that of hydrogen peroxide (H2O2).
Disulfur dibromide is the most stable sulfur bromide, however, its thermal stability is low. It is a toxic, orange/yellow liquid that smokes in air due to the reaction with water vapor. In the presence of moisture, disulfur dibromides reacts violently with oxidants, releasing bromide anion and sulfur oxides. In general, its reactivity resembles that of S2Cl2.
...
Wikipedia