Disulfite
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
disulfite
|
|
| Other names
metabisulfite ion
pyrosulfite |
|
| Identifiers | |
|
PubChem CID
|
|
| Properties | |
| S 2O2− 5 |
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
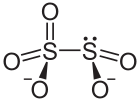
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the disulfite ion (metabisulfite ion) S
2O2−
5.
Surprisingly, and in contrast to disulfate (S
2O2−
7), disulfite ion (S
2O2−
5) has two directly connected sulfur atoms, and the structure is described as "thionite-thionate", or [O2S–SO3]2−, instead of the symmetrical form [O2S–O–SO2]2−.
The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3.
The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.
The disulfite ion is a dimer of the bisulfite ion (HSO−
3). It can arise from:
In aqueous solution, the disulfite ion is formed in minor amounts by dehydration of bisulfite in an equilibrium:
Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.
In fact, disulfite is the ion of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above:
...
Wikipedia
