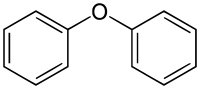
Diphenyl ether
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
1,1'-Oxydibenzene
|
|
| Other names
Oxydibenzene
Diphenyl ether Diphenyl oxide 1,1'-Oxybisbenzene Phenoxybenzene |
|
| Identifiers | |
|
101-84-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:39258 |
| ChEMBL |
ChEMBL38934 |
| ChemSpider |
7302 |
| ECHA InfoCard | 100.002.711 |
| UNII |
3O695R5M1U |
|
|
|
|
| Properties | |
| C12H10O | |
| Molar mass | 170.21 g·mol−1 |
| Appearance | Colorless solid or liquid |
| Odor | geranium-like |
| Density | 1.08 g/cm3 (20°C) |
| Melting point | 25 to 26 °C (77 to 79 °F; 298 to 299 K) |
| Boiling point | 121 °C (250 °F; 394 K) at 1.34 kPa (10.05 mm Hg), 258 °C at 100 kPa (1 bar) |
| Insoluble | |
| Vapor pressure | 0.02 mmHg (25°C) |
| -108.1·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | Aldrich MSDS |
| NFPA 704 | |
| Flash point | 115 °C (239 °F; 388 K) |
| Explosive limits | 0.7%-6.0% |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
3370 mg/kg (rat, oral) 4000 mg/kg (rat, oral) 4000 mg/kg (guinea pig, oral) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 1 ppm (7 mg/m3) |
|
REL (Recommended)
|
TWA 1 ppm (7 mg/m3) |
|
IDLH (Immediate danger)
|
100 ppm |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Diphenyl ether is the organic compound with the formula O(C6H5)2. The molecule is subject to reactions typical of other phenyl rings, including hydroxylation, nitration, halogenation, sulfonation, and Friedel–Crafts alkylation or acylation. This simple diaryl ether enjoys a variety of niche applications.
Diphenyl ether and many of its properties were first reported as early as 1901. It is synthesized by a modification of the Williamson ether synthesis, here the reaction of phenol and bromobenzene in the presence of base and a catalytic amount of copper:
Involving similar reactions, diphenyl ether is a significant side product in the high-pressure hydrolysis of chlorobenzene in the production of phenol.
The main application of diphenyl ether is as a eutectic mixture with biphenyl, used as a heat transfer medium. Such a mixture is well-suited for heat transfer applications because of the relatively large temperature range of its liquid state. A eutectic mixture [commercially, Dowtherm A] is 73.5% diphenyl ether (diphenyl oxide) and 26.5% diphenyl (biphenyl).
Diphenyl ether is a starting material in the production of phenoxathiin via the Ferrario reaction. Phenoxathiin is used in polyamide and polyimide production.
...
Wikipedia

