Diovan
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Diovan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697015 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Protein binding | 95% |
| Biological half-life | 6 hours |
| Excretion | Renal 30%, biliary 70% |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.113.097 |
| Chemical and physical data | |
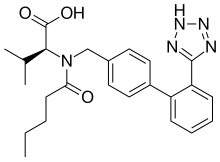
| Formula | C24H29N5O3 |
| Molar mass | 435.519 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Valsartan (trade name Diovan) is an angiotensin II receptor antagonist (commonly called an ARB, or angiotensin receptor blocker), that is selective for the type I (AT1) angiotensin receptor. Valsartan is mainly used for treatment of high blood pressure, congestive heart failure, and to increase the chances of living longer after a heart attack.
Valsartan is used to treat high blood pressure, congestive heart failure, and to reduce death for people with left ventricular dysfunction after having had a heart attack.
There is contradictory evidence with regard to treating people with heart failure with a combination of an angiotensin receptor blocker like valsartan and an angiotensin-converting enzyme inhibitor, with two major clinical trials (CHARM-additive and ValHeFt) showing a reduction in death, and two others (VALIANT and ONTARGET) showing no benefits, and more adverse effects including heart attacks.
In people with type II diabetes and high blood pressure or albumin in the urine, valsartan is used to slow the worsening and the development end-stage renal disease.
The packaging for valsartan includes a warning stating the drug should not be used with the renin inhibitor aliskiren in people with diabetes mellitus. It also states the drug should not be used in people with kidney disease.
Valsartan falls in FDA pregnancy category D and includes a black box warning for fetal toxicity. Discontinuation of these agents is recommended immediately after detection of pregnancy and an alternative medication should be started. The US labeling makes no recommendation regarding continuation or discontinuation of valsartan for breast-feeding mothers. The Canadian labeling does not recommend use by nursing women.
...
Wikipedia
